
In this retrospective, real-world study, we analyzed IBM MarketScan® Commercial Claims and Medicare Supplemental databases to assess the impact of flash CGM on diabetes-related events and hospitalizations in a cohort of 2,463 individuals with type 2 diabetes on short- or rapid-acting insulin therapy. Use of flash continuous glucose monitoring (flash CGM) improves glycemic control in type 1 and type 2 diabetes, which may result in lower risk for acute and chronic complications that require emergency services and/or hospitalizations. This website and the information contained herein is intended for use by residents of the United States.Suboptimal glycemic control among individuals with diabetes is a leading cause of hospitalizations and emergency department utilization. The product images are for illustrative purposes only. No use of any Abbott trademark, trade name, or trade dress in this site may be made without the prior written authorization of Abbott Laboratories, except to identify the product or services of the company. Other trademarks are the property of their respective owners.
Freestyle libre flash glucose monitoring system ndc full#
Review all product information before use or contact Abbott Toll Free ( 85) for detailed indications for use and safety information.įor full indications for use and safety information, see more here.įreeStyle, Libre and related brand marks are trademarks of Abbott Diabetes Care Inc. The built-in blood glucose meter is not for use on dehydrated, hypotensive, in shock, hyperglycemic-hyperosmolar state, with or without ketosis, neonates, critically-ill patients, or for diagnosis or screening of diabetes.

Sensor placement is not approved for sites other than the back of the arm and standard precautions for transmission of blood borne pathogens should be taken. The FreeStyle Libre system is not approved for pregnant women, persons on dialysis, or critically-ill population. The FreeStyle Libre system does not have alarms unless the sensor is scanned, and the system contains small parts that may be dangerous if swallowed. Check sensor glucose readings with a blood glucose meter when Check Blood Glucose symbol appears, when symptoms do not match system readings, or when readings are suspected to be inaccurate. WARNINGS/LIMITATIONS: Do not ignore symptoms that may be due to low or high blood glucose, hypoglycemic unawareness, or dehydration. The system is intended for single patient use and requires a prescription.ĬONTRAINDICATIONS: Remove the sensor before MRI, CT scan, X-ray, or diathermy treatment.
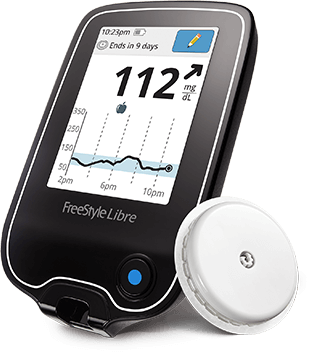
The FreeStyle Libre Flash Glucose Monitoring system is a continuous glucose monitoring (CGM) device indicated for replacing blood glucose testing and detecting trends and tracking patterns aiding in the detection of episodes of hyperglycemia and hypoglycemia, facilitating both acute and long-term therapy adjustments in persons (age 18 and older) with diabetes. Indications and Important Safety Information † Based on the sensor being replaced once every 10 days, and scanned at least once every 8 hours. * Fingersticks are required for treatment decisions when you see Check Blood Glucose symbol, when symptoms do not match system readings, when you suspect readings may be inaccurate, or when you experience symptoms that may be due to high or low blood glucose.


 0 kommentar(er)
0 kommentar(er)
